
Pipeline -Korrosion ist im tatsächlichen Arbeitsprozess sehr häufig. Wie viel wissen Sie dann darüber? Was sollten Sie tun, um es zu verhindern? In diesem Artikel wird über 10 Arten von Rohrkorrosion geführt und die Verhinderungswege für jeden von ihnen erzählt.
- Electrochemical corrosion
Reason: metal and electrolyte contact REDOX reaction.
Prevention methods:
Cathodic protection (sacrificial anode or impressed current).
Use anti-corrosion coatings (e.g., epoxy resin, polyethylene coating).

- Chemical corrosion
Reason: Metals react directly with chemicals such as strong acids and bases.
Prevention method:
Choose corrosion resistant materials (such as titanium alloy, Hastelloy).
Control the composition of the medium (e.g., adjust pH to reduce corrosive substances).

- Pitting (pitting)
Cause: Local chloride ions or sulfides cause small holes to form on the metal surface.
Prevention methods:
Use pitting resistant materials (such as 316L stainless steel, duplex steel).
Add corrosion inhibitors (such as molybdate, nitrate).

- Microbial Corrosion (MIC)
Reason: sulfate-reducing bacteria and other microorganisms metabolize acid or sulfide.
Prevention methods:
Clean pipes regularly and use fungicides or bioinhibitors.
Keep the pipe dry or control the temperature to inhibit microbial growth.
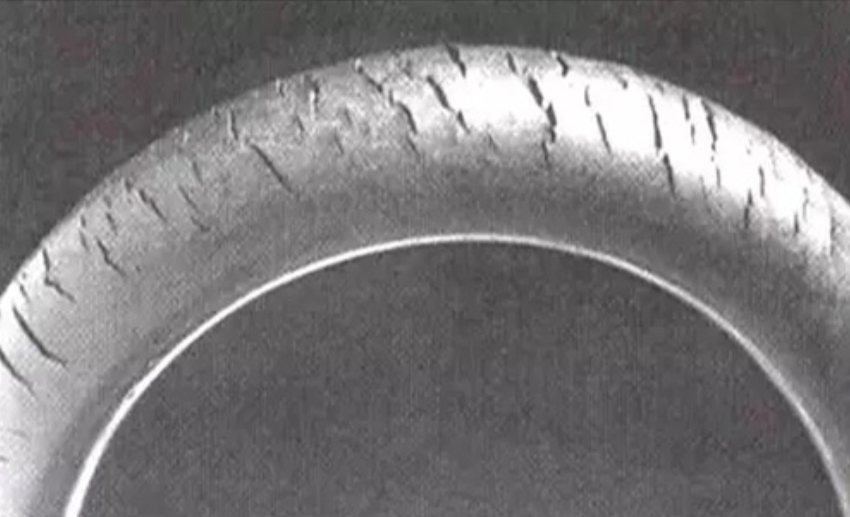
- Stress Corrosion Cracking (SCC)
Cause: Tensile stress and corrosive environment (such as chloride ions, high temperature).
Prevention methods:
Remove residual stress (annealing treatment).
Avoid using sensitive materials (such as austenitic stainless steel in chlorine environments).

- Uniform corrosion
Reason: The surface of the metal is uniformly eroded by the corrosive medium.
Prevention methods:
Increase wall thickness to reserve corrosion margin.
Use a coating or lining (such as a rubber lining).

- Crack corrosion
Reason: The concentration battery is formed by the retained corrosion medium in the gap of the pipeline.
Prevention methods:
Optimized design to avoid gaps (e.g. welded instead of bolted).
Fill gaps with sealant.

- Hydrogen brittle
Cause: Hydrogen atoms penetrate into the metal causing brittle fracture.
Prevention methods:
Control the hydrogen sulfide content in the medium.
Low strength steel or hydrogen trap alloy.

- Erosion corrosion
Cause: high-speed fluid impact damage the surface protective film.
Prevention methods:
Reduce flow rate or change direction design.
Use wear-resistant materials (such as silicon carbide coatings).

- Stray current corrosion
Cause: An external current (such as a tram track) triggers an electrolytic reaction.
Prevention methods:
Install drain device or insulation flange.
Strengthen insulation protection between pipeline and soil.

